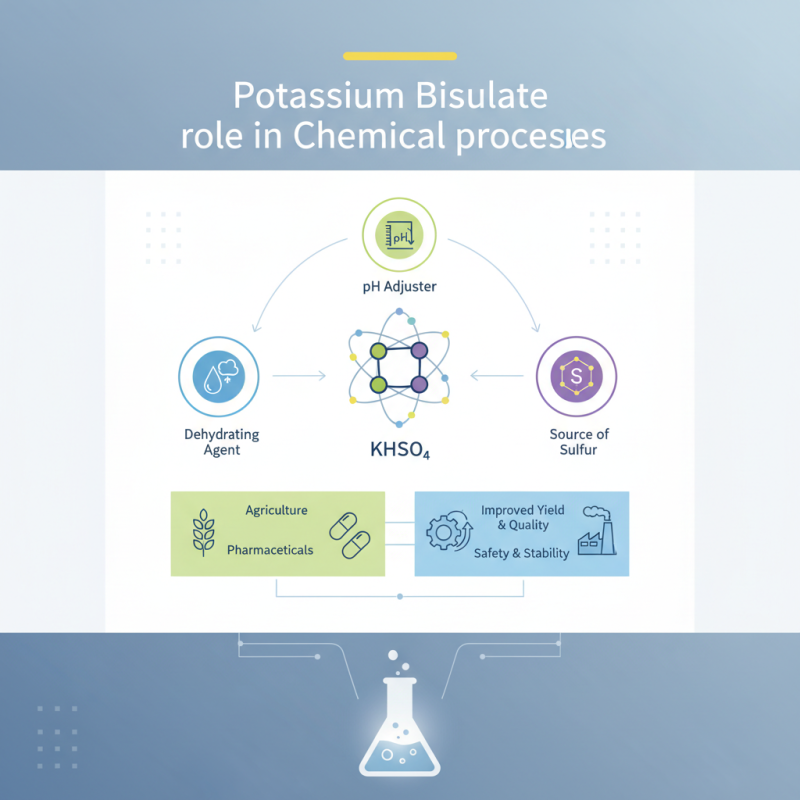
Potassium Bisulfate, a versatile compound commonly used in various chemical processes, plays a crucial role in enhancing efficiency and effectiveness in numerous applications. As industries increasingly seek reliable and economical solutions for their chemical reactions, the importance of Potassium Bisulfate becomes ever more prominent. This compound serves multiple purposes, including acting as a dehydrating agent, a pH adjuster, and a source of sulfur, thereby facilitating a range of chemical transformations.
The significance of Potassium Bisulfate extends across various fields, from agriculture to pharmaceuticals, indicating its vast applicability. It not only contributes to improving yield and quality in production processes but also ensures safety and stability during reactions. By understanding the unique properties and benefits of Potassium Bisulfate, manufacturers and researchers can harness its potential effectively, leading to optimized workflows and enhanced product quality.
In this article, we will delve deeper into the essential roles played by Potassium Bisulfate in chemical processes, exploring its characteristics, applications, and the advantages it offers to various industries. Understanding these aspects will provide valuable insights into why Potassium Bisulfate is regarded as an indispensable component in the world of chemistry.
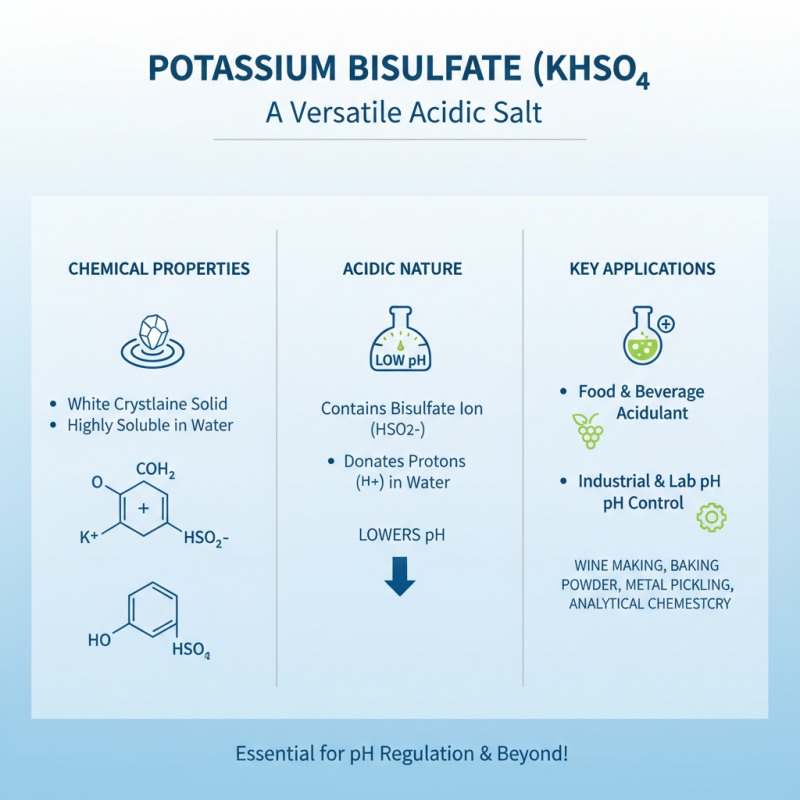
Potassium bisulfate, chemically represented as KHSO4, plays a crucial role in various chemical processes due to its unique properties. This white crystalline solid is highly soluble in water, making it an excellent acidulant in food and beverage applications. Its acidic nature arises from the presence of the bisulfate ion (HSO4−), which can donate protons in aqueous solutions, thereby lowering pH levels effectively. This characteristic is invaluable in numerous industrial and laboratory settings where pH control is critical.
In addition to its acidifying properties, potassium bisulfate exhibits hygroscopic behavior, meaning it can absorb moisture from the environment. This property is beneficial in processes that require specific humidity levels or where moisture control is paramount. Its use as a catalyst in various chemical reactions further emphasizes its functional versatility.
Tip: When handling potassium bisulfate, always wear appropriate protective gear, including gloves and goggles, to ensure safety, as it can irritate skin and eyes. Additionally, store the chemical in a cool, dry place to maintain its effectiveness and prevent clumping due to moisture absorption.
Potassium bisulfate, a versatile chemical compound, plays a significant role in various industrial processes. One of its primary applications is in the production of fertilizers, where it provides essential potassium and sulfur nutrients that enhance plant growth and yield. Additionally, potassium bisulfate is utilized in the food industry as a food preservative and acidulant, helping to maintain flavor and extend shelf life. Its ability to regulate pH levels makes it a valuable ingredient in beverage production, contributing to taste balance and stability.
In the realm of chemical manufacturing, potassium bisulfate is critical in synthesizing other chemicals and compounds. It serves as a dehydrating agent in the production of various organic and inorganic materials. Furthermore, its use in metal processing as a flux aids in facilitating the melting and purification of metals, ensuring the quality of the final product.
Tips: When handling potassium bisulfate, always wear appropriate personal protective equipment (PPE) to minimize exposure. Ensure that you are working in a well-ventilated area to avoid inhalation of dust. Additionally, consider conducting a risk assessment before using it in your processes to ensure compliance with safety regulations and best practices in handling chemicals.
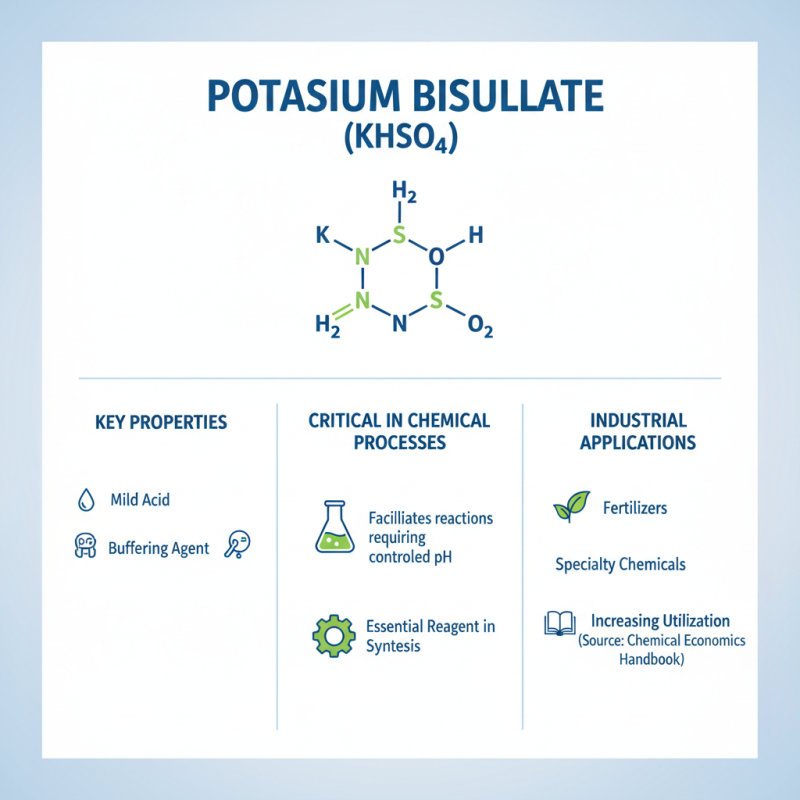
Potassium bisulfate (KHSO4) is critical in various chemical processes due to its ability to act as a mild acid and a buffering agent. In many industrial applications, KHSO4 serves as an essential reagent in the synthesis of chemicals, where its unique properties facilitate reactions that require controlled pH levels. According to a recent report by the Chemical Economics Handbook, potassium bisulfate is increasingly utilized in the production of fertilizers and specialty chemicals, highlighting its versatility and importance in modern chemical manufacturing.
The benefits of using potassium bisulfate in chemical reactions extend beyond merely controlling acidity. For instance, it is employed in the formulation of liquid fertilizers, allowing for improved nutrient delivery to plants by enhancing nutrient solubility. Research indicates that the use of potassium bisulfate can result in increased crop yields, with some studies showing improvements of up to 20% in specific crops when compared to conventional fertilizers. Furthermore, its efficiency in stabilizing pH levels in chemical processes contributes significantly to the reproducibility and reliability of reactions, ensuring consistent product quality.
Additionally, potassium bisulfate acts as a dehydrating agent in organic synthesis, enabling reactions that would be hindered by water. Its capacity to selectively remove water without decomposing sensitive compounds makes it invaluable in the synthesis of high-purity products. The growing trend towards greener chemistry also boosts the appeal of potassium bisulfate, as it is less hazardous compared to some of its alternatives, which aligns with the industry's shift towards sustainable practices. According to the latest data from the American Chemical Society, there is a rising demand for mild, effective chemicals like KHSO4, which meet rigorous safety and environmental standards while delivering optimal performance in chemical processes.
When handling potassium bisulfate, it’s crucial to adhere to safety and handling guidelines to ensure a safe working environment. First and foremost, wear appropriate personal protective equipment (PPE), which includes gloves, goggles, and lab coats to minimize exposure to skin and eyes. Inhalation of dust or vapors can cause respiratory discomfort, so using a fume hood or ensuring adequate ventilation in the workspace is vital. Additionally, it’s important to have safety data sheets (SDS) readily available, detailing the chemical properties and first-aid measures in case of exposure.
Proper storage of potassium bisulfate also plays a critical role in maintaining safety. It should be stored in a cool, dry place in tightly sealed containers to avoid moisture absorption, which can lead to the formation of corrosive substances. Be cautious when handling this chemical around incompatible materials such as strong bases or metals, as reactions can be hazardous. Spills should be addressed immediately following established protocols, using appropriate containment and disposal methods, ensuring the area is cleaned up promptly to prevent further risks. By following these safety guidelines, the risks associated with potassium bisulfate can be effectively managed.
When examining alternative chemical agents that can be used in processes typically involving potassium bisulfate, it is essential to consider both their effectiveness and the potential trade-offs. Potassium bisulfate is widely recognized for its role as an acidulant and a source of sulfur, which functions efficiently in various applications, including food preservation and chemical synthesis. However, alternatives such as citric acid and sodium bisulfate can be employed in similar contexts. Citric acid, for instance, is a natural compound that offers a milder taste profile and acts as a chelating agent, making it suitable for food-related applications, while sodium bisulfate serves as a strong acid and is often used in water treatment processes.
Despite their benefits, these alternatives may not always match the specific functionalities provided by potassium bisulfate. For example, while citric acid is safer and more environmentally friendly, its effectiveness in applications requiring a controlled pH may be limited in comparison. Sodium bisulfate, on the other hand, can present handling challenges due to its corrosive nature, which might necessitate additional safety measures. Therefore, when considering alternatives to potassium bisulfate, one must evaluate not only the functional attributes but also the handling characteristics and compatibility with existing processes to ensure optimal outcomes in chemical applications.
| Chemical Agent | pH Level | Oxidizing Power | Cost per Kg | Environmental Impact |
|---|---|---|---|---|
| Potassium Bisulfate | 4.0 - 5.0 | Moderate | $1.50 | Low |
| Sodium Bisulfate | 1.5 - 2.5 | Low | $1.20 | Medium |
| Calcium Sulfate | Neutral | Low | $0.80 | Low |
| Sodium Hydroxide | 12.0 - 14.0 | High | $2.00 | High |
| Sodium Carbonate | 9.0 - 11.5 | Moderate | $1.00 | Low |






